[Hui Cong Pharmaceutical Industry Network] "Drug quality, people's livelihood security" has increasingly become the focus of public opinion. The Party Central Committee and the State Council have also attached great importance to drug supervision work, and have repeatedly stressed that the production source is safe and effective, and the quality is controllable.

However, China's pharmaceutical production enterprises have a large number and wide distribution, and there are many varieties of drugs and many types of drugs. The production process is relatively complicated and professional. Due to the small number of supervisors and heavy tasks, the frequency of supervision is low, there are many blind spots, and there is a lack of a seamless supervision. The means, in practice, can only rely on the employee's consciousness, which makes the entire production process full of risks.
On January 6, 2017, the production record of Shanghai Qingan Pharmaceutical Group Suzhou Pharmaceutical Co., Ltd. was untrue. The lottery numbers of 151101, 151102, and 151103 were extracted from the extraction, sedimentation, filtration, and drying process. The signature time nodes of individual operators and the attendance time registered on the enterprise time sheet were inconsistent.
So, what is the source of the factors affecting the safety of pharmaceutical production?
The source of production can be summarized into two categories: First, the entrance and exit of the clean area; Second, the human body is the largest source of pollution. At present, some high-risk pharmaceutical manufacturers in China have begun piloting the control of high-risk clean-zone entrances and exits through radio frequency technology, and controlling the risks from the entrance of the clean-up area. Eliminate unauthorized personnel from entering and leaving the clean area, reduce the occurrence of cross-contamination caused by contact with the door opening device at the entrance to the clean area; in addition, during the production process, the human body is the largest source of pollution in the clean area and the most uncontrollable risk Factors, so the control of human pollution is also particularly important.
Policy drives product innovation and development:
With the accelerated implementation of the 2010 GMP certification standard for the pharmaceutical industry and the further improvement of personnel management requirements in the pharmaceutical industry, the life cycle of clean clothing consumables and the traceability management of products are increasingly valued.
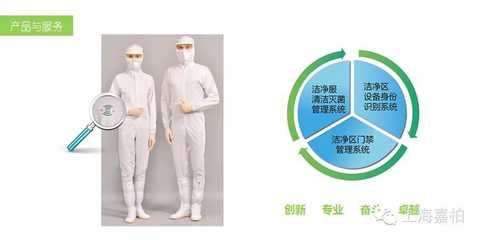
Gabriel Technology is investigating and collecting data from pharmaceutical companies. And in cooperation with a representative pharmaceutical factory, developed a "smart clean clothes and personnel integrated management system" - clean clothing information query and traceability system, to achieve uniform management of fabrics, styles and cleaning and sterilization times of clean clothes, At the same time, the cleaning, sterilization and life cycle of the clean use process are unified and electronically and efficiently managed. The system has obtained a number of patent authorizations from the State Intellectual Property Office.
At the same time, we saw the GMP requirements for the access control system in the inspection report of the British Food and Drug Administration (MHRA). According to MHRA's inspection defects made by a company, the access control system used to control personnel entering the GMP area (such as clean area, quality control area, storage area, stability test area) needs to be included in GMP management. At the same time, functional requirements for access control systems are also found in the FDA and China GMP regulations/guidelines: “Only authorized employees can enter facilities and facilities that are restricted to enter. Only confirmed and properly dressed personnel can Allow access to sterile production areas."
In the pharmaceutical production area, employees wearing safety equipment and electronic equipment (including wearing glasses, etc.) will have a safety impact on the environment. The entrance and exit control of the clean area is not like the traditional access control system. Achieve clean room access management. In addition, due to the particularity of the clean area, the current mainstream biometric technologies on the market, including fingerprints, palm prints, facial recognition, etc., are also difficult to apply to the management control of clean areas. In order to comply with relevant national laws and regulations and to meet the needs of the market. In the face of market defects, after years of accumulation and communication with pharmaceutical management personnel, Gabriel's research experts pioneered the management of clean areas by implanting high-temperature and corrosion-resistant RFID electronic tags in clean clothes. Device. The manager integrates access control functions and clean service management functions to fill the gap in the domestic market. Also obtained a series of national invention patents. It overcomes the difficulties that traditional access cards cannot be used in clean areas, and also solves the problem of clean clothing specification management.
Intelligent access control system:
Shandong Taibang Biological Products Co., Ltd., the only blood products company listed in the US. In the second phase of the project, Taibang Biotech invested heavily in the creation of a modern smart factory, in line with the corporate social responsibility of practicing conscience and rest assured medicine! Taibang Biotech entrusted JIABO Gabriel Technology to implement the second phase of the factory personnel intelligent access control system installation. After a month of intense heat fighting, the system installation test was completed and the acceptance was successful. Jiabo's leading products and efficient services are highly recognized by customers.
Through the distribution and use subsystem of the system software platform and the access control management subsystem, the system operator can conveniently manage the access of employees to the clean area, control the number of personnel in the clean area in real time, improve the efficiency of personnel control in the clean area, and promote the clean room operation room. Security management. At the same time, you can also inquire about the entry and exit records of personnel in the clean area. It reduces the difficulty of personnel management, fills in the gaps that can be changed by traditional manual records, and improves the level of information management.




Net service management function:
The intelligent clean clothes and personnel integrated management system dynamically monitors the process data of clean clothes, people, environment and equipment by implanting electronic tags into the clean clothes used in the sterile areas of pharmaceutical companies to establish a unique identity for each person in the workshop. Assist in user management and reduce contamination of clean room operations due to human factors. Through the electronic label of the clean clothes, we can control the access control system in the clean area to control the activities of people entering and leaving the clean area of ​​the workshop. In addition, we can record the cleaning and sterilization process of each clean clothes, and then control the use of clean clothes. The service life guarantees the use of clean clothes in accordance with the national GMP dress code; in addition, it can also be combined with the start-stop control of production equipment to simplify the authentication and authorization method of the production equipment system.
"Jiabo Technology, companionship" is our commitment to customers; "Quality comes from design, professionalism comes from concentration". This is our pursuit of products; "Be good now, do yourself, do your job, say It is necessary to do this.†This is our requirement for employees; “Cohesion and common progress; Huijiang River becomes the sea, and it is accumulated thousands of miles.†This is our advocacy for management. Looking forward to the future, we will continue to optimize the clean room and personnel intelligent management solutions in the clean area, we are confident in meeting the intelligent engineering needs of users.
Editor in charge: Yang Manman
Serving the industry with our best quality products, we manufacture, supply Coral Fleece slipper. This product has perfect fittings and made with excellent quality. It ensures durable finish and water resistant. It does not give uncomfortable feel at first wear. These are offered in various sizes, depending upon the need of the client. We have been applauded for this superior quality product every time we have delivered.
White Hotel Slippers,Plush Hotel Slipper,Close Toe Coral Fleece Slipper,Hotel Plush Slipper
Yangzhou Lansun Slipper Co.,Ltd , https://www.lansunslippers.com